Summary
Staphylococcus aureus is among the most prevalent pathogens in skin and soft tissue infections, with treatments becoming increasingly compromised by the emergence of methicillin-resistant S. aureus (MRSA). Vanderbilt researchers have developed a combination photodynamic therapy (PDT) for targeting MRSA infections in skin that is not only effective but also highly specific and less susceptible to resistance, adding a much needed therapy to our quickly depleting arsenal against this pathogen.
Addressed Need
Skin and soft tissue infections, often caused by S. aureus, account for over 14 million ambulatory care visits per year, with antibiotic-resistant MRSA strains causing tens of thousands of deaths and billions of dollars in clinical costs. As antibiotic resistance becomes increasingly more prevalent, we risk being unable to fight this once treatable disease. This technology provides a powerful new weapon for filling that gap.
Technology Description
This technology leverages a combination of therapeutics and PDT, a therapy commonly used in treating skin cancer and acne, to specifically treat MRSA infections. First, the patient receives 1) a monoclonal antibody that is conjugated to a near-infrared photosensitizer and 2) a small molecule that activates the production of blue light photosensitizers specifically in Gram positive bacteria like S. aureus. Near-infrared and blue light are then directed at the infection site, producing reactive oxygen species that kill the pathogens.
Competitive Advantages
This approach is highly specific, avoiding the negative off-target effects of broad-spectrum antibiotics. This therapy is also dramatically less susceptible to resistance due to the combination of multiple targets and the growth-essential nature of the blue light photosensitizers. As an additional benefit, the antibody can be administered intravenously to protect against systemic infection, which has a high mortality rate of 24% in hospital-acquired cases.
Stage of Development and IP Status
This technology has been validated in vitro and in vivo. We are seeking commercial partners to further develop it for clinical applications.
Patents: US20220080036. US9867879B2. Other PCT patent applications pending.
Publication: Antimicrobial Agents and Chemotherapy 2021
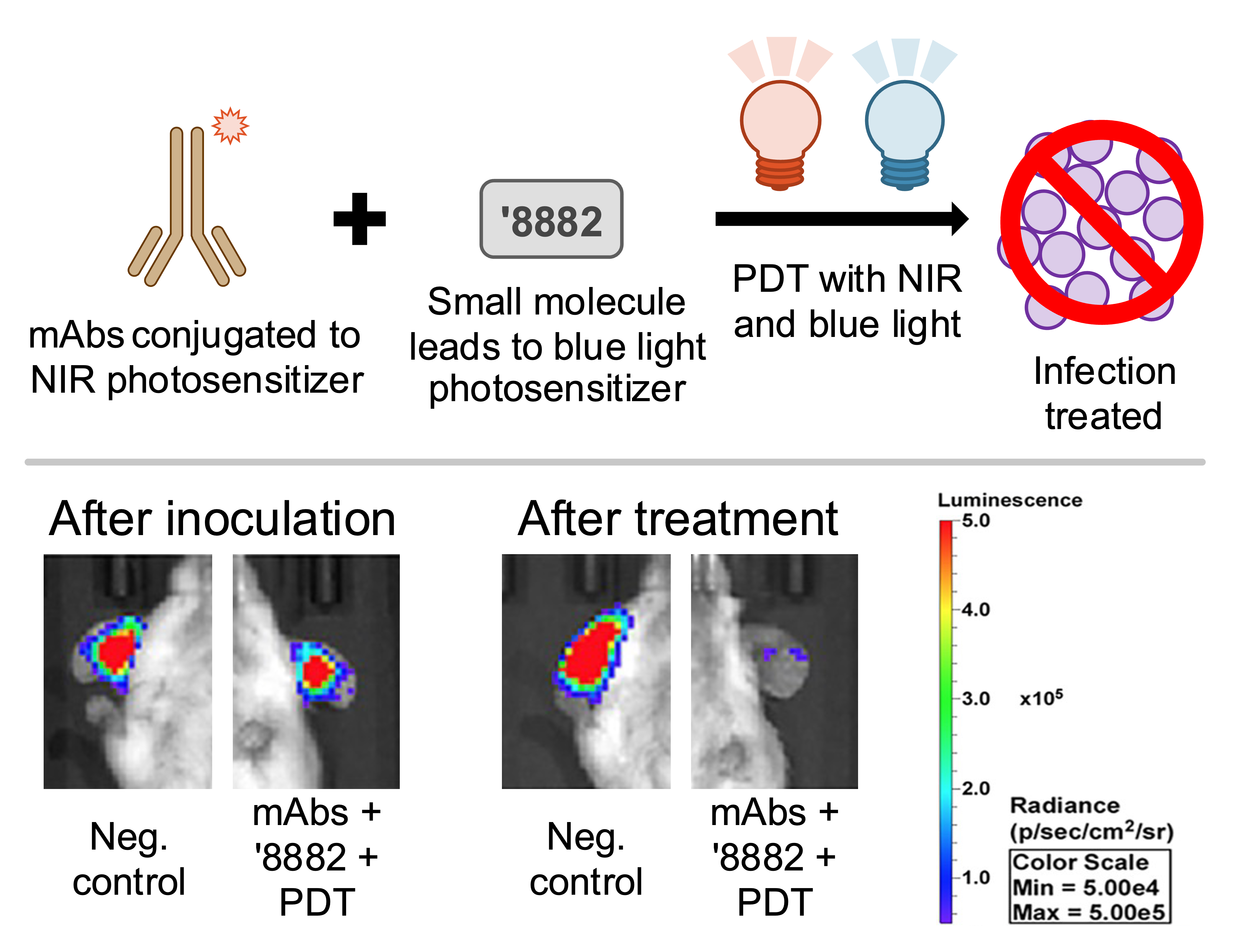
Top: Co-administering the antibodies and small molecule developed by our researchers enables PDT treatment of S. aureus infections. Bottom: in vivo mouse model demonstrates effective treatment of luminescent MRSA skin infection.




