Summary
Vanderbilt researchers have developed an injectable drug delivery vehicle using microparticles (MPs) that can deliver cargos in the eye for at least 6 weeks while also playing a therapeutic role in reducing inflammation.
Addressed Need
Targeted delivery of anti-inflammatory drugs could be used to treat many ocular diseases, including glaucoma and traumatic optic neuropathy, two blindness-inducing conditions associated with inflammation of the optic nerve. While therapeutics can be effectively delivered to this tissue by injection, the rapid clearance of free drugs from the intravitreal space necessitates recurring injections that increase costs, patient inconvenience, and the risk of further damage. Intravitreally injected MPs can be used to release drug cargos over time, but many of these are made from materials that exacerbate inflammation, including the clinical gold standard poly(lactic-co-glycolic acid) (PLGA).
Technology Description
To overcome these challenges, Vanderbilt researchers have developed MPs with tunable properties using propylene sulfide (PS) and ethylene sulfide (ES) copolymers. Being hydrophobic themselves, these MPs can be loaded directly with hydrophobic cargos, many of which cannot be delivered to target tissues using conventional approaches; or loaded with hydrophilic or charged cargos using novel preparation methods. This polymer backbone becomes hydrophilic when oxidized, releasing its cargo while removing reactive oxygen species (ROS) from the tissue. As drug cargo for treating inflammation of the optic nerve, the inventors have also modified the anti-inflammatory cytokine erythropoietin (EPO) to reduce potentially dangerous side effects.
Competitive Advantages
Whereas injections of free EPO are cleared from the intravitreal space in a matter of days, the PPSES MPs have been demonstrated to provide over 6 weeks of sustained drug release in the eye (Fig. A). While the commonly used PLGA exacerbates inflammation, PPSES acts as an antioxidant, significantly reducing ROS in the environment when administered alone or with EPO cargo (Fig. B). Data from electrophysiological tests indicate that this two-pronged therapeutic effectively improves visual function in mouse models of multiple ocular diseases (Fig. C).
Stage of Development and Intellectual Property Status
The inventors have now developed 3rd generation MPs with increased drug delivery durations and improved visual performance. They have validated these and prior versions of their technology in vitro using endotoxin and drug release kinetics assays; and in vivo using mouse models for glaucoma and traumatic optic neuropathy. We are seeking commercial partners to further develop this technology for clinical applications.
Patents: WO2023205175. Additional patent application has been filed.
Publication: Journal of Controlled Release, 2021
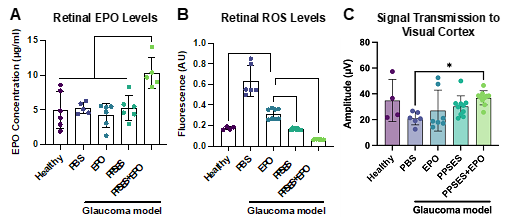
Figure: Biochemical and electrophysiological data from a mouse model for glaucoma demonstrate effective treatment of inflammation in the eye using EPO-loaded PPSES MPs. A MPs provided sustained EPO delivery for 6 weeks, yielding higher retinal EPO levels at the end of the study. B PPSES scavenges ROS from the eye, resulting in near-normal levels of ROS when administered alone and below-normal levels of ROS when loaded with EPO. C Electrophysiological data, such as this VEP data measuring ganglion cell function, indicate that intravitreal PPSES MPs loaded with EPO improve visual function.




