Summary
Recent FDA approvals have spurred the demand for viral vector products, prompting the need for more efficient bioproduction methods. In this context, Vanderbilt researchers have engineered a new cell line with significantly enhanced viral production capabilities compared to traditional cell lines. This innovation is expected to lead to substantial cost savings in viral vector production and improved viral products, two key advantages in the industry.
Addressed Need
Viral vector manufacturing is a multibillion-dollar industry, with an expansion of manufacturing facilities being needed to meet the ever growing demand for applications in gene therapy and vaccine design. Using current technologies, these increases in demand could only be produced with costly increases in manufacturing facilities and resources. However, by leveraging a unique genetic modification in a commonly used cell line, Vanderbilt scientists have developed a technology that can help bridge this gap by producing viral vectors with higher titers and improved transduction efficiency.
Technology Description
By genetically increasing the expression of a proprietary gene in immortal HEK293T cells, Vanderbilt researchers have created a cell line that protects viral products from cellular degradation, leading to a significant boost in the production and effectiveness of viral vectors. Expression of this gene could also be manipulated via the administration of a small molecule or biologic, enabling diverse methods of implementation to best meet manufacturing needs.
Competitive Advantage
Compared to unmodified HEK293T cells, these genetically modified HEK293T cells can produce, on average, a 263-fold increase in viral titer, with those vectors displaying an 84-fold increase in transduction efficiency. Given this novel cell line's increased productivity and transduction efficiency, this technology can improve the effectiveness of existing viral products and significantly reduce viral vector manufacturing costs. Furthermore, because this modification impacts the production capabilities of the host cell itself, it can be employed to enhance the production of a wide range of mammalian viruses using multiple vector types and sizes.
Stage of Development
This technology has been validated in vitro for viral vector production and delivery efficiency using lentivirus and retrovirus. Testing is underway for adenovirus and adenovirus-associated virus constructs. We are seeking commercial partners to help scale this technology for industrial use.
Intellectual Property Status
Patents: Patent application has been filed.
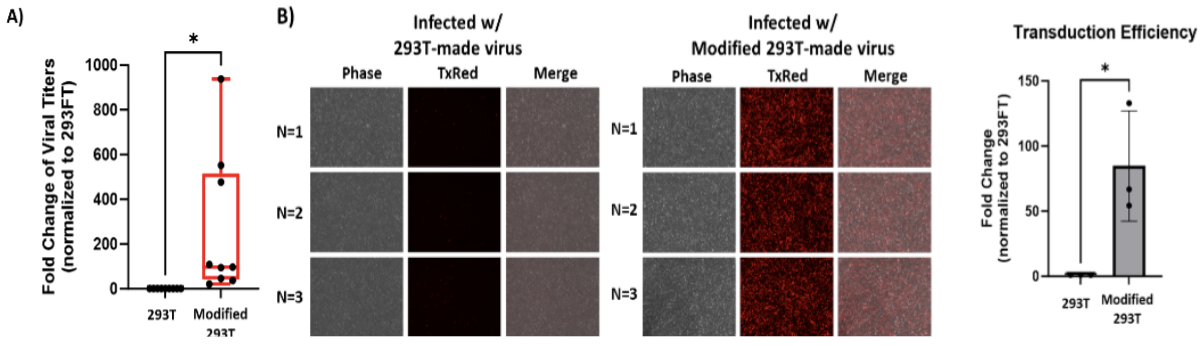
A) Genetically modified 293T cells produce significantly higher titers of viral vectors than unmodified 293T cells. B) In vitro viral transduction assay demonstrates the significantly improved efficiency of viral vectors produced by modified 293T cells, as indicated by an 84-fold increase in fluorescence.




