Summary
Despite the success of immune checkpoint inhibitors (ICI) like anti-PD-1 in treating other cancers, these therapeutics have not been demonstrated to effectively treat acute myeloid leukemia (AML). Vanderbilt researchers have identified PD-1H as a potential target for treating AML, opening the door for effective therapy using an ICI molecule.
Addressed Need
AML accounts for 33% of all adult leukemias [ACS]. While there are multiple treatments for AML currently in the clinic, the 5-year relative survival rate for patients 20 years and older is only 28% [NCI], indicating the need for better treatments. Vanderbilt scientists have identified a new target for treating AML and other blood disorders like myeloid dysplastic syndrome.
Technology Description
Scientists at Vanderbilt have discovered that AML could be treated by blocking the novel target PD-1H using an ICI molecule. Whether administered on its own or together in combination with anti-PD1, anti-PD-L1, and/or anti-CTLA4, data from mouse models revealed that anti-PD-1H antibodies can effectively preserve the T cell-powered immune response against cancer cells.
Competitive Advantages
PD-1H is a relatively recently discovered and understudied immune checkpoint molecule, such that targeting PD-1H presents an opportunity for novel therapies for AML. Data indicate that combining PD-1H targeting with existing ICI therapies (e.g., anti-PD-1) leads to synergistic effects, enhancing treatment efficacy to deliver 100% survival in model mice. In addition, unlike PD-1 or PD-L1,PD-1H is highly expressed in AML residing in the bone marrow, enabling effective targeting of AML from the source.
Stage of Development
This technology has been validated in vivo using mouse models. We are seeking commercial partners to develop it for clinical applications.
Patents: Pending US patent application.
Publication: J Clin Invest, 2023.
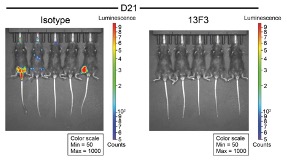
PD-1H KO mice were transplanted intravenously with AML cells expressing full length PD-1H and treated with anti- PD-1H or isotype control antibody every 4 days. Mice were assessed for in vivo AML cell proliferation using bioluminescence imaging (top, at 21 days), the quantification of which (bottom) showed that anti-PD-1H treatment dramatically reduced the growth of disseminated PD-1H expressing AML cells.




