Summary
While medical diagnostics today typically tradeoff between speed and sensitivity, Vanderbilt researchers have developed a diagnostic platform that is both rapid and quantitative by coupling lateral flow paper-based tests with color changing porous silicon membranes. This platform can be adapted to detect a wide range of biological and chemical targets, enabling the rapid measurement of various biomarkers.
Addressed Need
Rapid testing is critical to improving disease diagnosis and monitoring therapeutic treatment plans. However, current rapid diagnostic tests are often not sensitive enough for early-stage diagnosis and lack the necessary quantification for disease staging and routine monitoring of biomarker levels. Meanwhile, more sensitive and quantitative assays, like those used clinically, are expensive, require lab facilities and expertise, and have slow turnaround times. A rapid diagnostic platform that is sensitive and quantitative would launch the next generation of medical diagnostics, enabling faster and more informed treatment – not only in the clinic but even in the home.
Technology Description
This technology combines the sensitivity of porous silicon with the speed of lateral flow assays to create a point-of-care diagnostic platform capable of rapidly and accurately measuring biomarkers. Like a pregnancy or COVID-19 test, this device can detect a particular biomarker from a drop of body fluid. However, rather than presenting a yes or no answer, the test strip undergoes a concentration-dependent color change that can be measured with a smartphone camera.
Competitive Advantages
1. Quantitative analysis: Concentration-dependent changes in color enable the sensitive measurement of analytes.
2. Sensitive detection: The large surface area created by the silicon pores allows efficient binding of low-concentration targets, enabling more accurate detection.
3. Rapid diagnosis: The paper-based substrate draws the fluid through the porous silicon membrane, dramatically reducing testing times.
4. Versatility: This platform can be adapted to measure a wide range of biological and chemical targets.
5. Portability: The finger-sized design and compatibility with simple equipment allow these tests to be administered in the doctor’s office, at home, or on the road.
Stage of Development
This technology has been validated in the lab, and we are seeking commercial partners to further develop it for clinical and consumer applications.
Intellectual Property Status
Patents: Patent application has been filed.
Publication: A manuscript is being prepared.
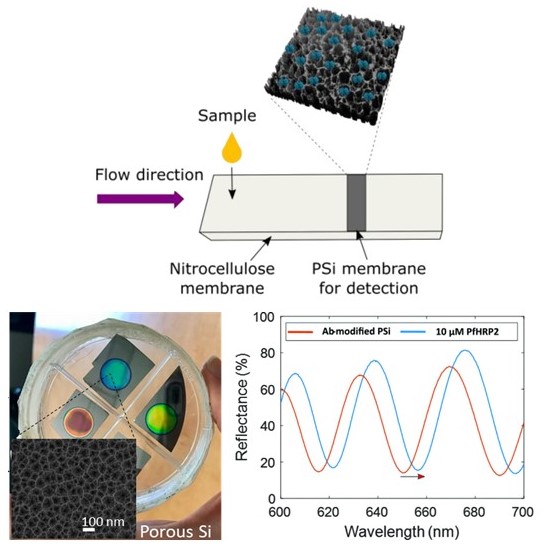
Top: Analytes are drawn through the strip as in a pregnancy test. Bottom: The porous silicon changes color based on the concentration of the biomarker (left), which can be quantified for a more informed response (right).




